Background:
Immunotherapies have shown dramatic anti-cancer efficacy in pediatric hematopoietic malignancies. Despite initial disease clearance, relapse rates are high, demanding product improvement. Manufacture of engineered cellular immunotherapies involves expansion of effector cells from patient or donor peripheral blood mononuclear cells (PBMCs) ex vivo prior to patient infusion. Expansion methods in pre-clinical studies of engineered T-cell therapies often call for activating T cells from whole PBMCs using anti-CD3 and anti-CD28 co-stimulation. However, clinical production of T-cell therapies has shifted towards selecting CD4+ and CD8+ cells prior to activation due to higher product efficacy seen in clinical trial. Many acute myeloid leukemias (AML) are CD4+, introducing the risk of blast contamination during CD4+ selection. We questioned whether CD8+ T cells alone could be used to manufacture our clinical T cell product and sought to determine how selection affected our T cells. Understanding the biological impact of manufacturing changes between pre-clinical and clinical production is imperative for clinical translation.
Methods:
We have engineered T cells to secrete a CD123xCD3 binding bispecific engager molecule (CD123-ENG) to treat AML. Primary T cells were isolated from PBMCs, selected with microbeads, activated, and transduced with replication incompetent retroviral vectors. Unselected T cells were compared to CD4+, CD8+, or CD4+ and CD8+ selected T cells. T-cell transduction was confirmed with flow cytometry. Expression and secretion of CD123-ENG was confirmed with ELISA. Antigen-specific T-cell activation was determined by quantifying cytokine secretion in the presence of CD123+ target cell lines. Short-term cytotoxicity was examined in vitro against CD123+ target cell lines in 18-hour bioluminescence assays. T-cell serial cytotoxicity and persistence were tracked in 10-day serial stimulation studies using an IncuCyte live cell imaging system. Cytokines present during T-cell manufacture and serial stimulation were detected and quantified with Luminex multiplex assays. Immunophenotype and exhaustion markers were analyzed using flow cytometry. In vivo anti-AML cytotoxicity and T-cell persistence were determined in an AML xenograft model.
Results:
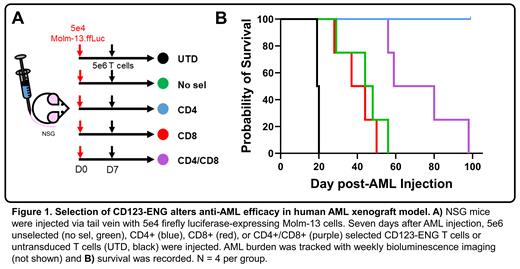
In our studies, unselected, CD4+, CD8+, and CD4+/CD8+ selected CD123-ENG T cells showed no differences in activation, cytotoxicity, or immunophenotype when evaluated in short-term assays. Mice harboring human AML treated with CD4+/CD8+ selected CD123-ENG T cells had improved survival compared to mice treated with unselected and CD8+ selected CD123-ENG T cells. CD4+ T cells alone provided the best survival advantage. In addition, CD4+/CD8+ selected T cells had longer persistence when challenged by serial target cell stimulations in vitro. T cells expanded from whole PBMCs were exposed to cytokines likely to emanate from non-T cells during early manufacture. These cytokines were characteristic of those produced by myeloid cells in an inflammatory environment, and may explain functional differences observed in vivo.
Conclusions:
Durable anti-cancer response in patients is associated with T-cell persistence and CD4+/CD8+ pre-selection of CD123-ENG may therefore improve remission duration in patients. CD4+ and CD4+/CD8+ selected T cells provided the longest survival in vivo, dissuading us from further investigating CD8+ CD123-ENG as an AML treatment. We are actively exploring additional mechanistic determinants of the observed phenotypic differences with and without selection. Optimizing cellular immunotherapy will improve patient outcomes. It is essential that engineered cellular research products intended for clinical translation be produced by the same methods used for clinical manufacture to increase the predictive power of pre-clinical data. The use of biological correlatives to predict T-cell product function will better support successful translation.
Acknowledgements:
This research was funded in part by NCI Contract No. HHSN26120150003I/HHSN26100077.
Disclosures
Bonifant:Dohme, Inc.: Research Funding; NA: Other: Patents pending on use of engineered cellular therapies for cancer; Sharpe: Research Funding; Merck: Research Funding; Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal